Vaska’s complex, or trans-carbonylchlorobis(triphenylphosphine)iridium(I) is one of the most studied organometallic complexes in which its studies paved way for the development of transition metal complexes for homogenous catalyst applications. Beside the complex’s application in catalytic hydrogenations of unsaturated hydrocarbon functionalities, it’s main use in organic synthesis is for the selective reduction of amides into highly substitued amines that are rather difficult to synthesize using traditional approaches.
In the Dixon group’s enantioselective total synthesis of (+)-Incargranine A, a triphenylphosphite derivative of Vaska’s complex with TMDS as the reductant was employed for a chemoselective amide reduction as a key step:
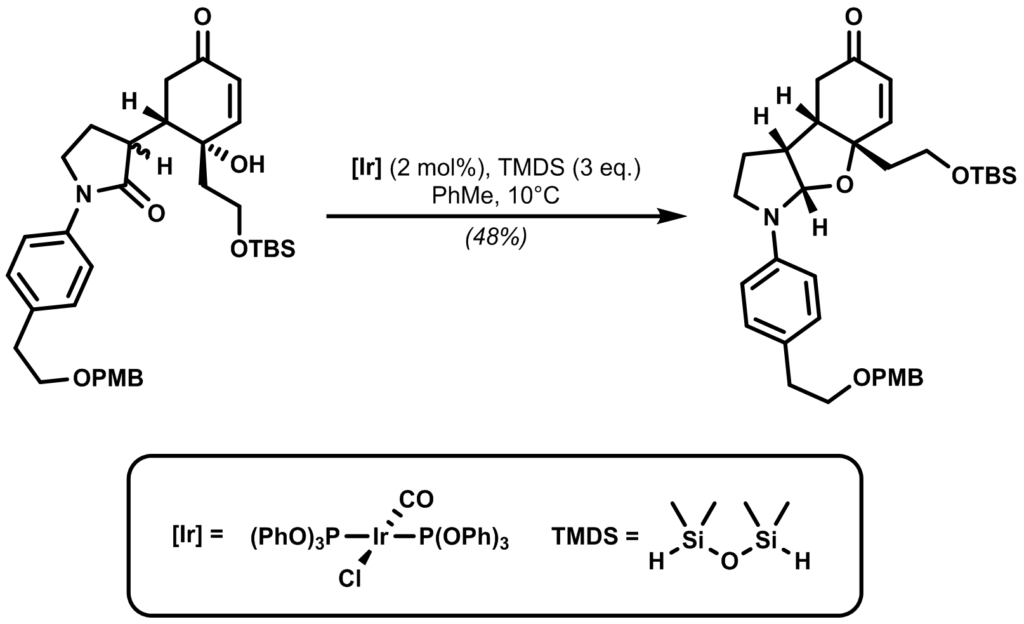
Identify the reactive intermediates involved in this reaction along with the full mechanism.
As a bonus, when this cyclic hemiaminal was exposed to 4N HCl, the final target (+)-Incargranine A was identified in the reaction mixture:
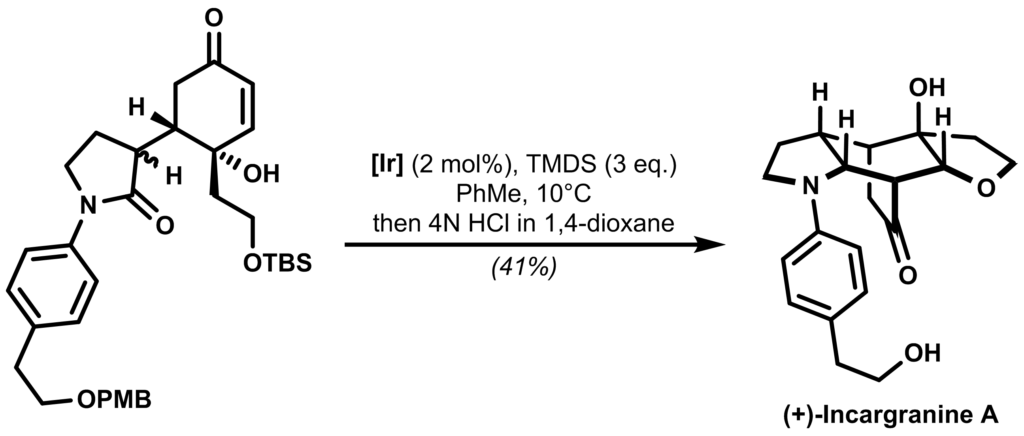
Propose a plausible mechanism for the reaction.
For reference:
Anna, Biallas, P., Benjamin, & Dixon, D. J. (2023).
“Enantioselective Total Synthesis of (+)‐Incargranine A Enabled by Bifunctional Iminophosphorane and Iridium Catalysis.”
Angewandte Chemie International Edition, 63(2).
doi: 10.1002/anie.202314308
hmm… it is so complicated mechanism but i can tell you one hypothesis.
First, the iridium catalyst makes complex with carbonyl part and hydroxide. The complex undergoes oxidative addition and TMDS can reduce the carbonyl part.
That’s all.
Sincerely.
PJM
hi~