Radical cyclizations offer a credible method for the synthesis of 5 or 6 membered rings. Carefully designed, it also enables the synthesis of multiple rings in one single synthetic step under mild and neutral conditions and thus have been utilized in a variety of synthetic schemes. The underlying reactivity of radical (or anionic) cyclization is usually well-predicted by Baldwin’s rules:
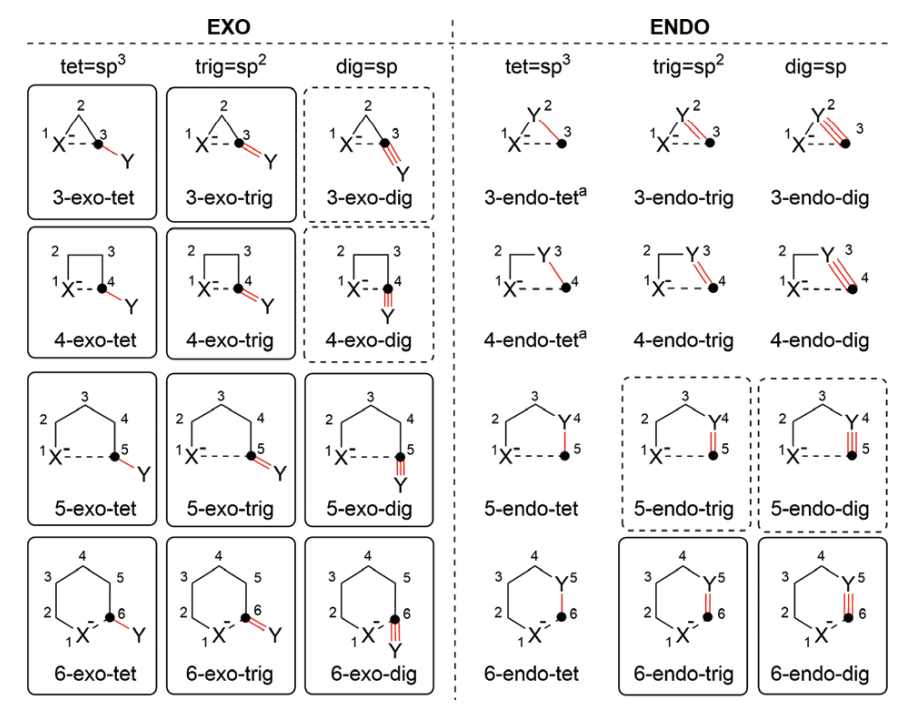
“The Baldwin rules: revised and extended.”
Wiley Interdisciplinary Reviews: Computational Molecular Science, 6(5), 487–514. doi:10.1002/wcms.1261
For larger rings (n>6), the reactivity trend is not strictly established – but the usual logic is that they are not favored over closures of smaller ring sizes albeit some specific reaction conditions may enhance its feasibility. An unique example of such larger-ring-cyclizations being favored over standard ring closures was reported in a 1998 JACS paper:
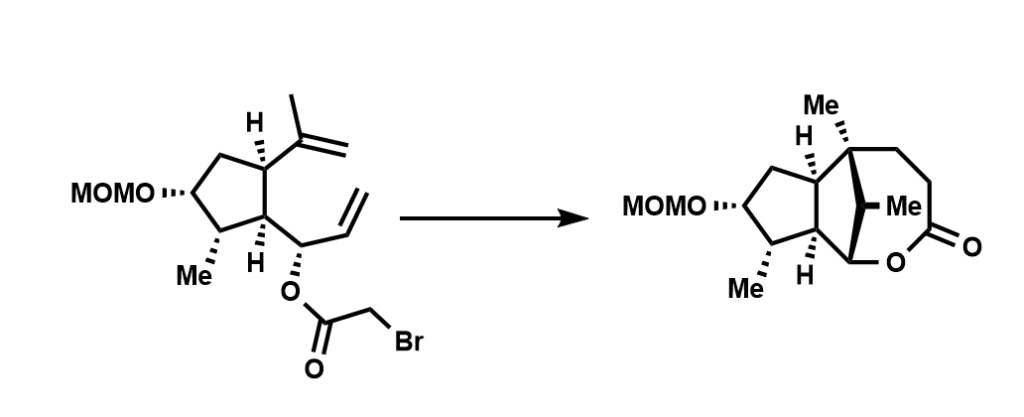
The authors isolated and characterized the product shown above under standard radical cyclization condition employing the classic tributyltin-AIBN combination. The expected outcome was a tricyclic γ-lactone via 5-exo/7-endo tandem radical cyclizations but they obtained a bridged 8-membered ring instead.
What was the original product that the authors envisioned? What is the mechanism for the product shown above? Name the shown cyclization in the standard Baldwin notation. What could account for the favorability of this larger ring closure?
For reference:
Lee, E., Yoon, C. H., Lee, T. H., Kim, S. Y., Ha, T. J., Sung, Y., Park, S., Lee, S. (1998).
“8-Endo Cyclization of (Alkoxycarbonyl)methyl Radicals: Radical Ways for Preparation of Eight-Membered-Ring Lactones.”
Journal of the American Chemical Society, 120(30), 7469–7478.
DOI: 10.1021/ja980908d