Malaria is one of the deadliest diseases that has remained unsuccumbed to modern medicine and treatment despite tremendous ongoing research, and is responsible for an estimated 600,000 deaths solely in the year 2022. Organic endoperoxides, namely artemisinin, the molecule that won the 2015 Nobel prize in physiology or medicine (recipient: Tu Youyou) is known to be particularly effective against malaria parasites. The mechanism of action putatively involves the heme-mediated decomposition of the endoperoxide bridge to produce open shell C-centered reactive intermediates that further interferes with endocellular machineries inside the parasite. The malaria parasite depends on unusually iron-rich hemes for survival which is derived from the proteolysis of host cell hemoglobin and thus shows increased affinity for organic peroxides compared to healthy cells.
In a 2004 paper by Kobayashi, the group synthesized a model endoperoxide in order to investigate their decomposition under Fe(II) ions. The following three products were isolated upon reacting the model compounds with FeSO4 as below:
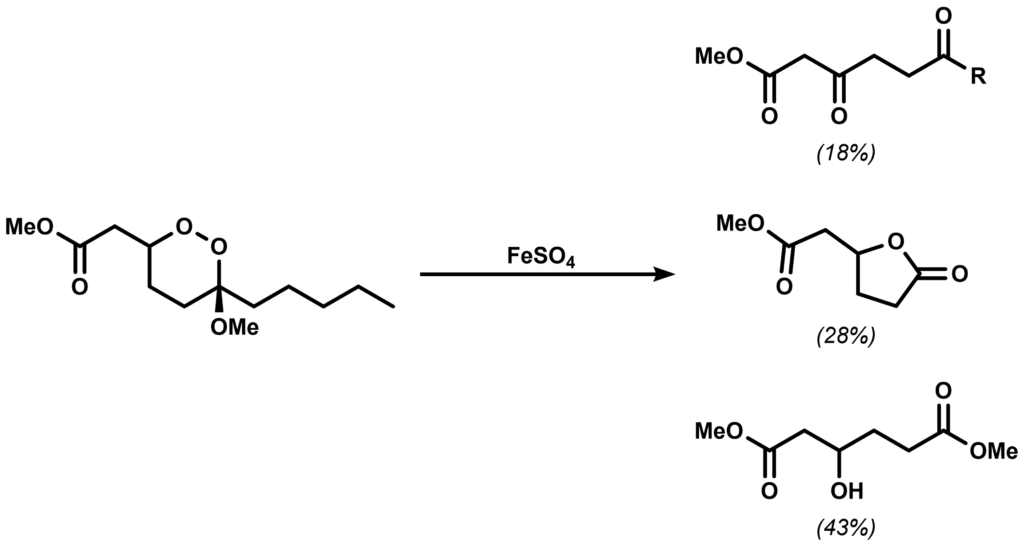
Give a plausible mechanism for the Fe(II)-mediated decomposition above.
For reference:
Murakami, N., Kawanishi, M., Itagaki, S., Horii, T., & Kobayashi, M. (2004).
“Synthesis of a bioprobe for elucidation of target molecule of spongean anti-malarial peroxides.”
Bioorganic & Medicinal Chemistry Letters, 14(13), 3513–3516.
DOI: 10.1016/j.bmcl.2004.04.086