Triquinanes are a family of tricyclic sesquiterpene compound composed of three angularly/linearly-fused cyclopentane rings. Among them, linearly fused triquinanes (or linear triquinanes) stood the test of time along with numerous syntheses and continues to be a common, but elegant target for total synthesis. In their paper mentioning some unusual reactivities of intramolecular [3+2] cycloaddition of trimethylenemethane (TMM) diradicals, Lee and coworkers from Korea Advanced Instituted of Science and Technology (KAIST) reported an intriguing synthetic approach towards the three linearly fused cyclopentane rings of triquinane natural products. Some of the representative linear triquinane natural products and Lee’s model transformation is shown as such:

Upon heating the shown diazabicycloheptene in THF, two products of different ring systems formed in separable mixtures. Disregarding the nature of substituent R, provide a reasonable mechanism for the mentioned cyclization.
Since their investigation of such [3+2] cycloadditions were initially explored in order to achieve a synthetic route towards another natural product with a different ring system, Conidiogenone B, the original cycloaddition also deserves a honorable mention:
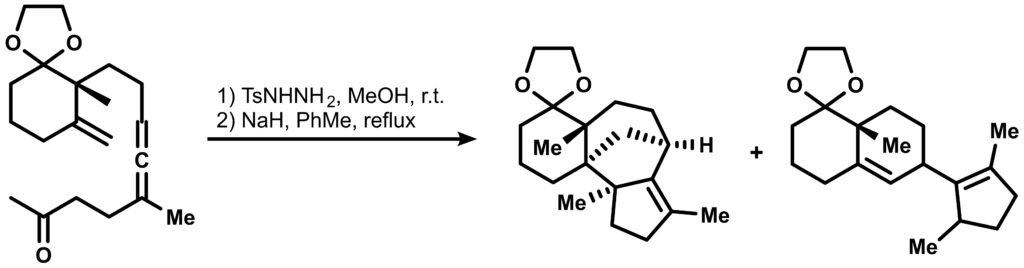
Draw a concise mechanism for the tandem cyclization above.
For reference:
Kim, M. J., Lee, S., Kang, T., Baik, M.-H., & Lee, H.-Y. (2020).
“Unexpected Selectivity of Intramolecular [3+2] Cycloaddition of Trimethylenemethane (TMM) Diyl toward Total Synthesis of Conidiogenone B”
European Journal of Organic Chemistry, 2020(5), 609–617.
DOI: 10.1002/ejoc.201901700