Domino reactions, or Tandem reactions are cleverly designed sequence of two or more individual reactions such that the former product becomes the starting material of the next reaction and so on. To borrow Tietze’s words from one of his review articles on domino reactions:
… a domino reaction is a process involving two or more bond-forming transformations (usually C-C bonds) which take place under the same reaction conditions without adding additional reagents and catalysts, and in which the subsequent reactions result as a consequence of the functionality formed in the previous step. A substrate with several functionalities which undergo a transformation individually in the same pot is not a domino reaction.
Tietze, L. F. (1996). Domino Reactions in Organic Synthesis. Chemical Reviews, 96(1), 115–136. doi:10.1021/cr950027e
Somfai’s group disclosed a concise approach towards (+)-Dehaloperophoramidine in 2017, completing the construction of a complex hexacyclic architecture in just 8 steps. The last two steps of the synthesis are shown below:
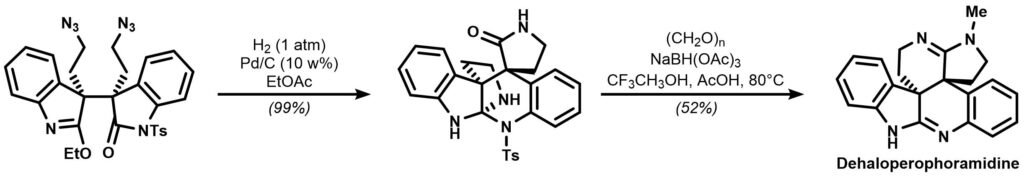
Draw a plausible mechanism for the two domino reactions!
For reference:
Hoang, A., Popov, K., & Somfai, P. (2017).
“An Efficient Synthesis of (±)-Dehaloperophoramidine.”
The Journal of Organic Chemistry, 82(4), 2171–2176.
doi:10.1021/acs.joc.6b02969