Marine ladder toxins, or Marine polycyclic polyethers are well known in the organic chemistry community for their repetitive cyclic ether linkages and bioactivity. Usually interrupting the voltage-gated sodium ion channel’s function, many of these molecular monstrosities have drawn significant interest among synthetic and medicinal chemists alike. Some of the representative natural products are illustrated below:

Synthesis of Marine Polycyclic Polyethers via Endo-Selective Epoxide-Opening Cascades. Marine Drugs, 8(3), 763–809.
More than two decades ago, West’s group from University of Utah developed an simple, iterative synthetic approach enabling the construction of mutiple 6-membered polycyclic polyethers using a controlled decomposition of diazo compounds via transition metal catalysts. The cyclization step yields an allyl-substituted endocyclic carbonyl, which could be further manipulated into an pendant diazoketone – allyl ether combination thus enabling another TM-catalyzed cyclization that also gives the same functional group duo as the starting material. This step could be iterated multiple times to prepare larger polycyclic ethers of desired number of rings:
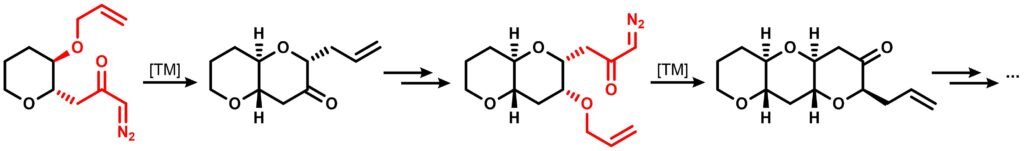
Give a mechanistic explanation regarding the TM-catalyzed cyclization step. The authors used rhodium(II) acetate and copper(II) hexafluoroacetylacetonate or trifluoroacetylacetonate as the catalyst.
Upon further inspection, West’s group found out that the alkyl group of the pendant ether affects the cyclization step’s product ratio. Consider the comparison of allyl and benzyl substituted experiments below:
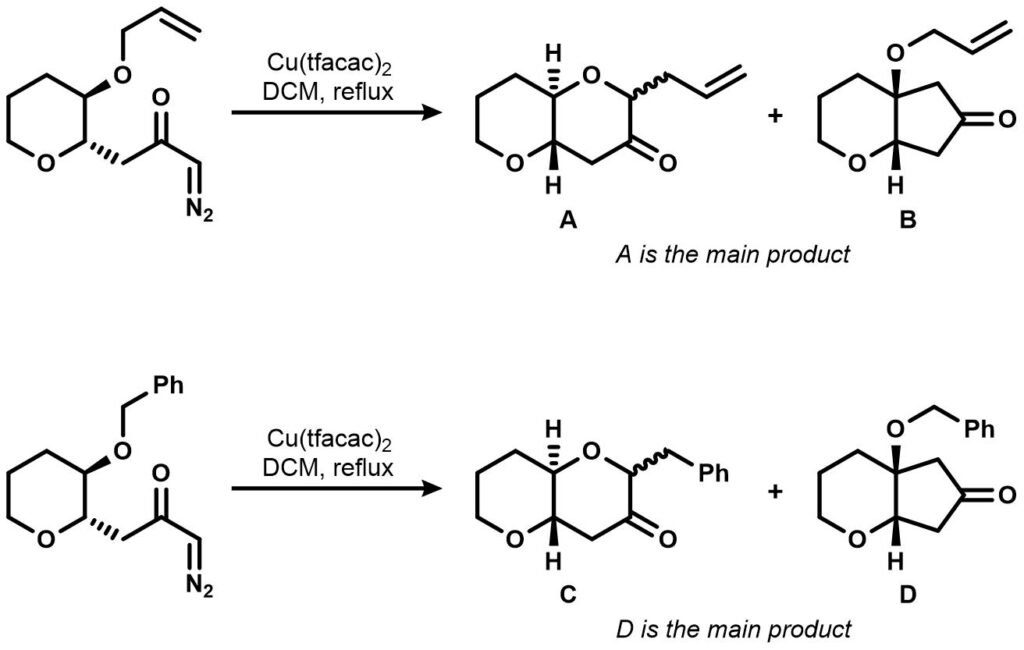
Which mechanism yields the 6/5 ring product? What can be inferred about the selectivity of the reaction from the product ratio above?
Besides the fact that this sparking method yields multiple polyether rings in considerably few (and high yielding) steps, I highly suggest you read the whole paper, as their mechanistic reasoning process and corresponding reaction designs raises some valuable considerations.
For reference:
Marmsäter, F. P., Vanecko, J. A., & West, F. (2002).
Cyclic oxonium ylides: building blocks for iterative synthesis of polycyclic ethers.
Tetrahedron, 58(10), 2027–2040.
doi:10.1016/s0040-4020(02)00051-0