Sometimes the most seemingly trivial transformations are the ones that bugs synthetic chemist the most. For example, during their synthesis of Gelsemoxonine, a spirocyclic alkaloid of the gelsemium family, the Fukuyama group needed to transform an α,β-unsaturated aldehyde to a fully saturated carboxylic acid ester. An otherwise straightforward manipulation proved to be extremely stubborn probably due to the steric hindrance provided by the bicyclic core, a situation commonly encountered for highly bridged, densely functionalized compounds.
After extensive experimentation, the group disclosed a rather unorthodox combination of reagents for the transformation: trimethylsilyl cyanide (TMSCN) and DBU. With concomitant trapping of the intermediate with allyl alcohol, the α,β-unsaturated aldehyde was successfully converted into its saturated ester, which was followed by a 4-step sequence that ultimately incorporated the long wished nitrogen atom into the molecule.
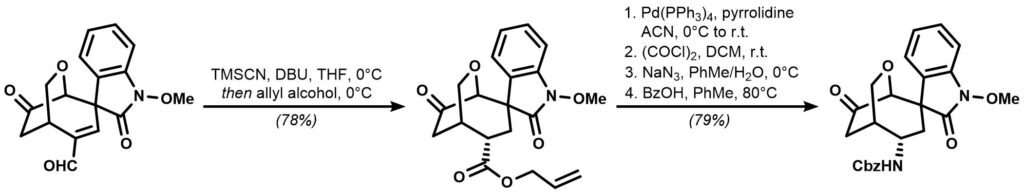
Draw and identify all the synthetic intermediate involved. Give a detailed mechanism for the first transformation.
For reference:
Shimokawa, J., Harada, T., Yokoshima, S., & Fukuyama, T. (2011).
Total Synthesis of Gelsemoxonine.
Journal of the American Chemical Society, 133(44), 17634–17637.
doi:10.1021/ja208617c