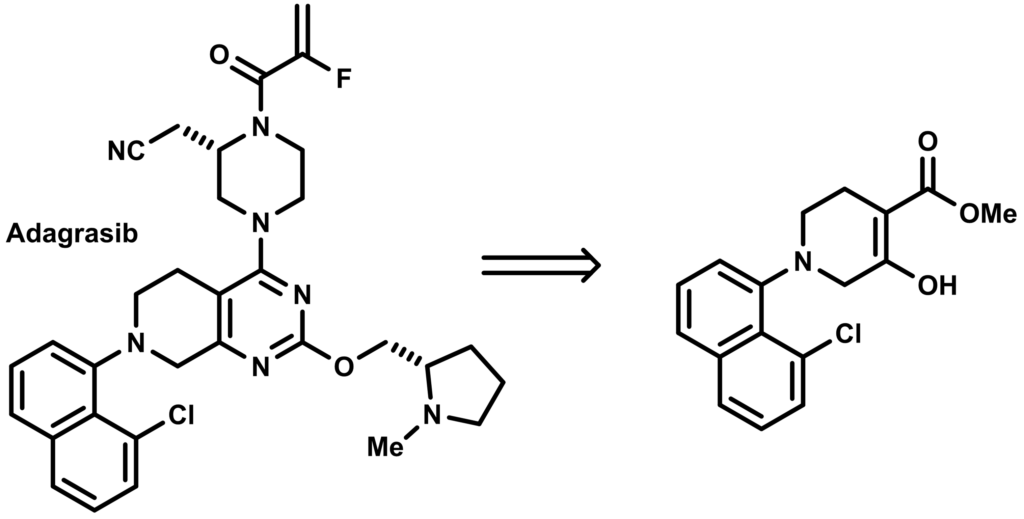
Adagrasib is a KRASG12C inhibitor approved for treating non-small cell lung cancer (NSCLC) with a specific KRAS gene mutation. It works by selectively targeting and inhibiting the mutated KRAS protein, thereby slowing cancer cell growth. The structure of adagrasib and its pivotal synthetic intermediate, a heterocyclic methyl ketoester flanked with a chloronaphthyl group can be shown below:
The first-generation synthesis towards adagrasib starts with a successive double alkylation of 8-chloronaphthalen-1-amine. The resulting Weinreb amide is treated with KHMDS to trigger a base-mediated intramolecular Dieckmann condensation and thus yields our methyl ketoester.
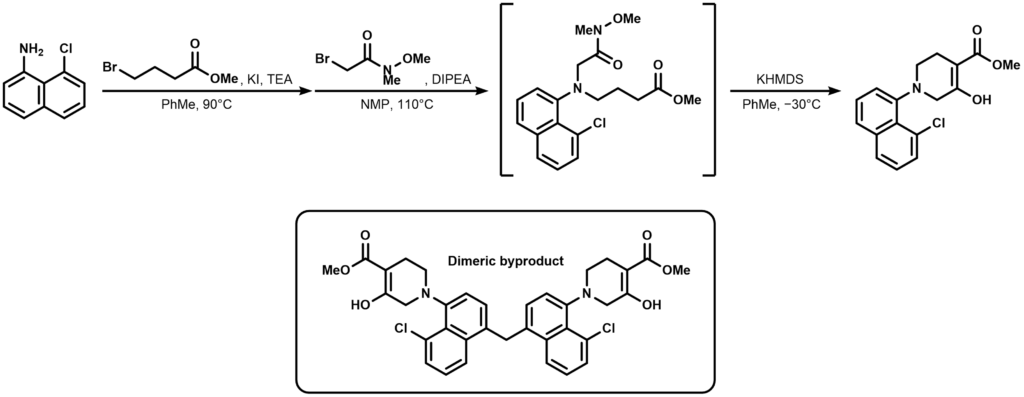
An interesting dimeric byproduct was observed during the reaction as shown in the box above. The structure is a methylene-bridged dimer of the reaction product connected via the 4-position of the naphthalene ring. Unfortunately the byproduct turned out to be quite stubborn towards purification procedures and the first generation route had to be abandoned for a new synthetic scheme.
What is the mechanism of this byproduct formation and what is the origin of the methylene bridge?
For reference:
Chen, C., Lu, Z., Scattolin, T., Chen, C., Gan, Y., & McLaughlin, M. (2023).
“Synthesis of Adagrasib (MRTX849), a Covalent KRASG12C Inhibitor Drug for the Treatment of Cancer.”
Organic Letters, 25(6), 944–949.
DOI: 10.1021/acs.orglett.2c04266
Lu, Z., Chen, C., Paymode, D. J., Gan, Y., Scattolin, T., Sahar Roshandel, Dong, W., Yu, S., Stéphane Lemeune, Snead, D. R., & Chen, C. (2024).
“Adagrasib’s Second-Generation Synthesis: Transitioning from Route Scouting to Optimization.”
Organic Process Research & Development, 28(8), 3128–3142.
DOI: 10.1021/acs.oprd.4c00024