The Diels–Alder reaction, first reported in 1928, is undeniably one of the most powerful synthetic methodologies known. Involving a diene and a dienophile, it is a pericyclic reaction that yields a cyclohexene moiety. Numerous modified analogues have since been developed and applied in areas ranging from complex natural product synthesis, polymerization, nanoparticle surface functionalization, in vivo biomolecule conjugation and more.
Although it is usually known that the DA reaction proceeds under thermal conditions (stated by the Woodward-Hoffmann rules), an exotic photochemical variant is also known as the “photo-DA reaction”. The transformation below is an early example of one reported by the Rawal group in 1999:
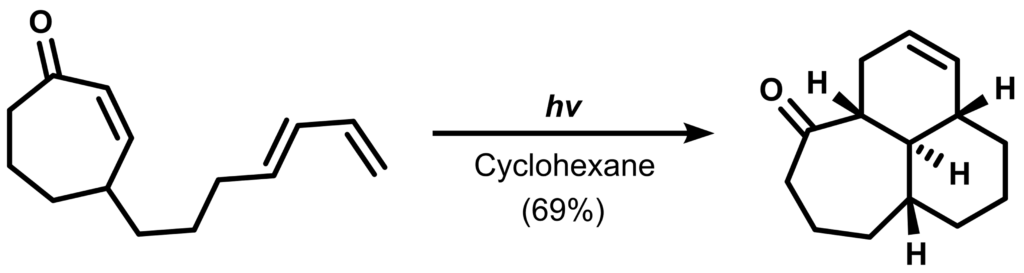
Why does this DA reaction require light? What is peculiar about the reaction product when compared to traditional thermal DA reactions? Propose a reaction mechanism for this reaction.
For reference:
D. Hilary, V. H. Rawal (1999)
“The Intramolecular Diels-Alder Reactions of Photochemically Generated trans-Cycloalkenones”
Journal of the American Chemical Society. 1999, 121, 10229-10230
DOI: 10.1021/ja992287