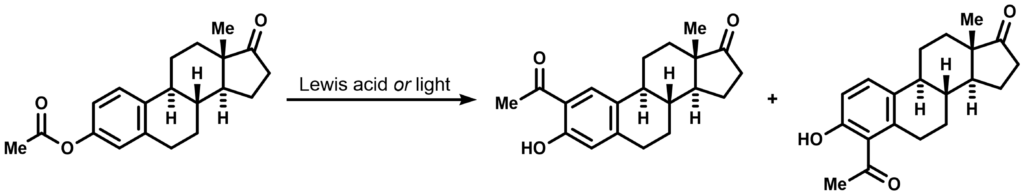
Fries rearrangement is a powerful reaction that facilitates direct acylation of arenes from O-acyl derivates of phenolic moieties. It provides a useful entry to aryl ketones since O-acylations of phenolic OH are usually more straightforward than selective acylation of an arene. Traditionally the reaction is performed under Lewis acidic conditions employing harsh reagents such as AlCl3 or SnCl4, but its milder photochemical derivant is also widely known and utilized (the photo-Fries rearrangement). An example of an orthodox Fries rearrangement performed on a steroidal sex hormone (estrone acetate) is provided below:
Strategic applications of the Fries-rearrangement in non-aromatic systems have shown some utility in the field of natural product total synthesis as well. In a classic synthesis of Phomoidride B, a.k.a. CP-263,114 performed by the Shair group in 2000, the transannular pseudoester ring system was installed via a tandem Lewis acidic Fries rearrangement-deprotection-cyclization sequence:
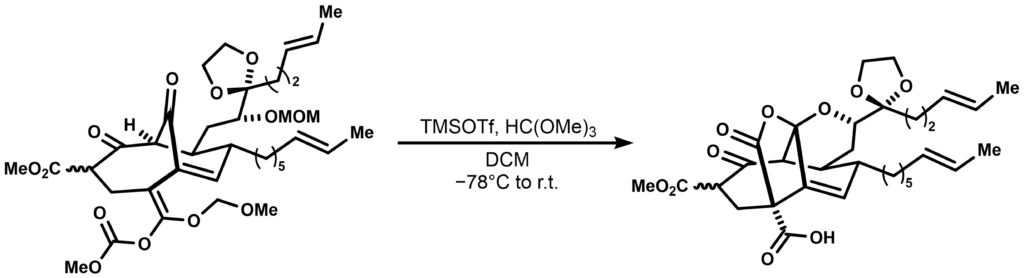
Such reactions highlight the fact that a well-designed rearrangement sequence can enable elegant, and selective transformations even for rather complex, highly advanced synthetic intermediates. Deduce the reaction mechanism for the tandem reaction above. What could be the significance of HC(OMe)3 in the reaction?
For reference:
Chen, C., Layton, M. E., Sheehan, S. M., & Shair, M. D. (2000).
“Synthesis of (+)-CP-263,114.”
Journal of the American Chemical Society, 122(30), 7424–7425.
DOI: 10.1021/ja001958x