Skeletal editing, or more precisely, ring atom editing has recently been a rising topic in synthetic organic chemistry since the emergence of various photocatalysts and their enabling reactions. It involves a sequence of exotic mechanisms dealing with single-atom deletion or addition to a ring (usually unsaturated and heterocyclic) and has the advantage to offer selective late-stage manipulations of complex natural products or synthetic intermediates.
Very recently the Nicewicz group published a highly photochemistry-reliant synthesis route for some of the stemoamide natural products. One of the non-photochemical steps involved a seemingly deceiving ring editing reaction, transforming a pyridinium ring to a fused formylpyrrole moiety:
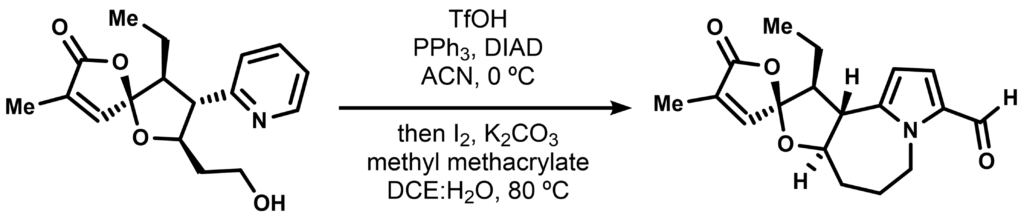
The reaction was first tested on a simpler substrate as such and the non-isolated bicyclic intermediate is shown below.
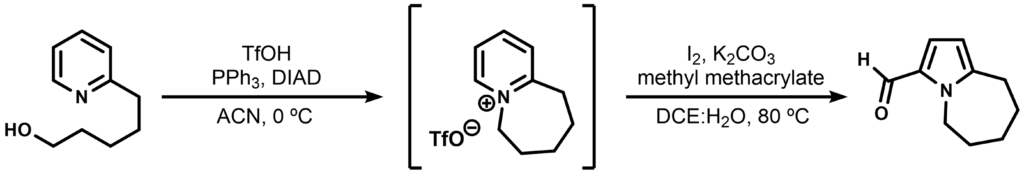
Suggest a mechanism for this ring-editing transformation.
For reference:
Akkawi, N. R., & Nicewicz, D. A. (2025).
“Photochemically Enabled Total Syntheses of Stemoamide Alkaloids.”
Journal of the American Chemical Society (2025), 147(18), 15482–15489
doi: 10.1021/jacs.5c01788