Three-membered rings, such as cyclopropanes, epoxides, aziridines, oxaziridines… show enhanced – or sometimes unexpected – reactivities compared to other rings with similar atomic composition but larger ring sizes. This makes these strained functionalities exotic synthetic surrogates for classical functional groups and when adequately utilized during synthesis, they can greatly enhance the elegance and conciseness of a synthetic route. Last year, the Soós group published a synthetic route towards a complex pentacyclic natural product Hunterine A that highlights the strategic employment of a skeletal rearrangment of an aziridine and an epoxide moiety.
Aziridine was introduced quite early in the synthesis during a tandem cyclization reaction yielding a tricyclic intermediate:
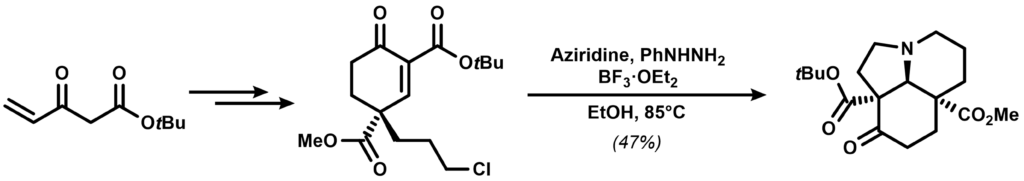
The intermediate was further elaborated into an indole containing a pendant epoxide that was exposed to acid, completing the synthesis:
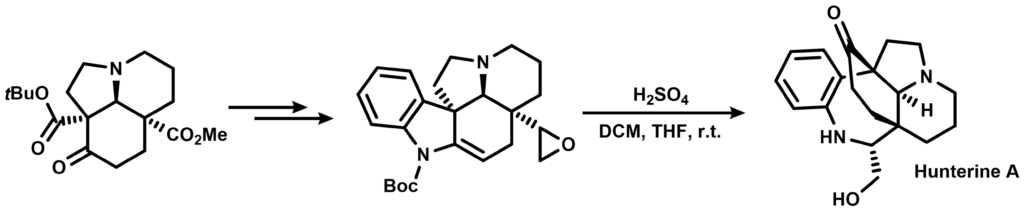
Suggest a reaction mechanism for the two transformations above.
For reference:
Zsigulics, B., Angyal, P., Mészáros, B. B., Daru, J., Varga, S., & Soós, T. (2024).
“Bioinspired synthesis of (–)‐Hunterine A: Deciphering the key step in the biogenetic pathway.”
Chemistry – a European Journal. 31(10)
doi: 10.1002/chem.202404501