Alkaloids isolated from the genus Daphniphyllum has puzzled and mesmerized organic chemists for for than three decades owing to their structural diversity and vibrant bioactivity. They belong to a family of more than 320 natural triterpenoids and have served as a long-standing synthetic target and a testing ground for newly developed methodologies. Qiu and co-workers published a divergent synthesis route towards (−)-daphenylline and (−)-himalensine A in 2021, featuring a common pentacyclic synthetic intermediate accessed from natural (+)-Carvone:
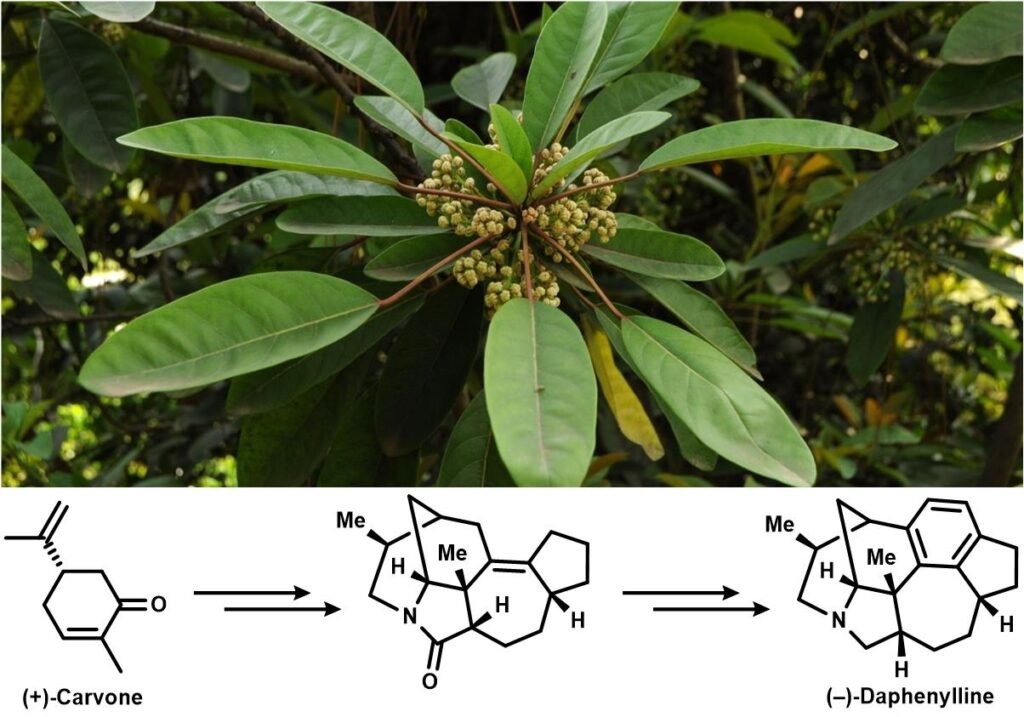
The intermediate amide then can be subjected to two different reaction sequences to yield either daphenylline or himalensine A:
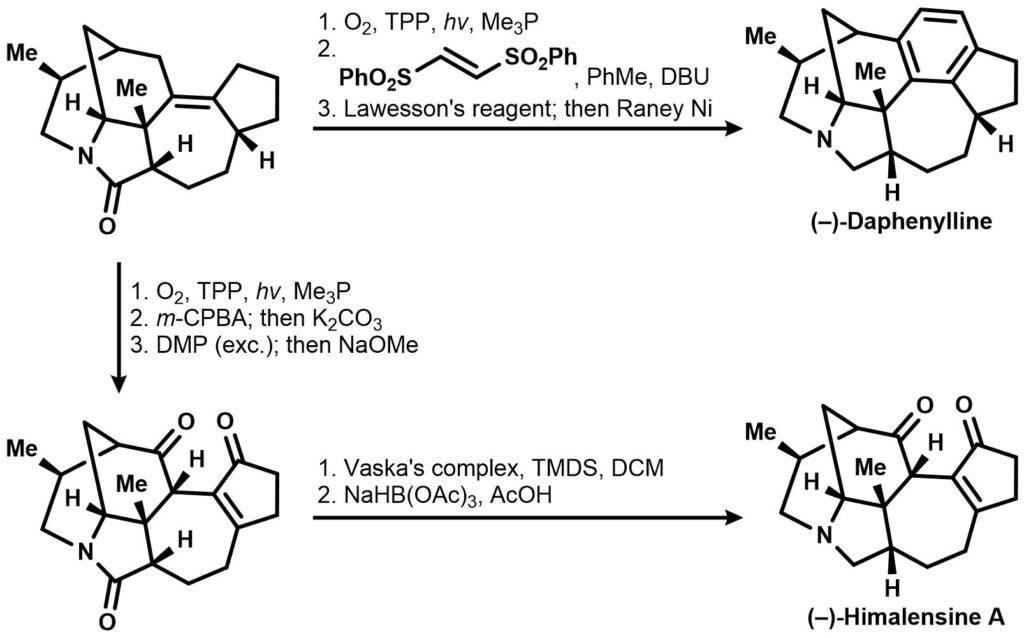
Identify all the synthetic intermediates and draw reaction mechanisms for the given set of reactions above.
For reference:
Wang, B., Xu, B., Xun, W., Guo, Y., Zhang, J., & Qiu, F. G. (2021).
“A General Strategy for the Construction of Calyciphylline A‐Type Alkaloids: Divergent Total Syntheses of (−)‐Daphenylline and (−)‐Himalensine A.”
Angewandte Chemie International Edition, 60(17), 9439–9443.
doi: 10.1002/anie.202016212