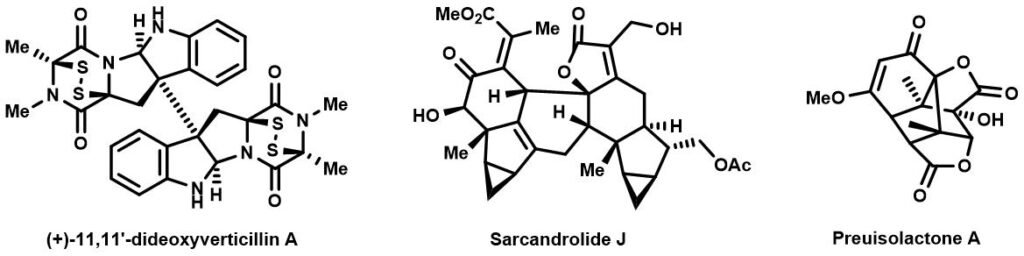
Dimeric structures and their corresponding monomers in natural products are sometimes hidden in plain sight, but sometimes the two monomeric motifs are quite difficult to pinpoint if a series of highly sophisticated reactions take place while merging the two. Some of the representative dimeric natural products are shown below, some more obviously “dimeric” than others. Can you identify the two monomers for each of the compounds?
It is therefore wise to first construct a monomer and then aim for a suitable dimerization reaction when synthetically approaching such compounds. In the Stoltz group, a very concise (4 steps!) approach was unveiled towards the synthesis of an another dimeric natural product isolated from Uvaria grandiflora, grandifloracin. Their synthesis commenced from the fully reduced form of salicylic acid, salicylic alcohol.
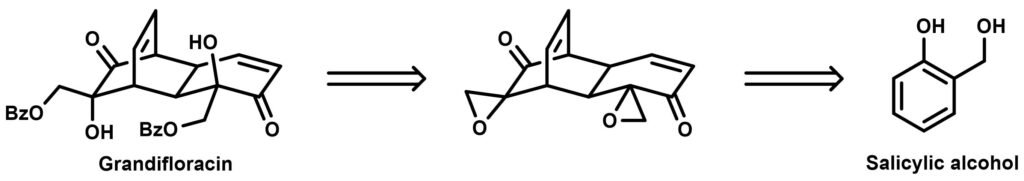
Astonishingly, the dimeric intermediate shown in the center was obtained with a single oxidation step utilizing salicylic alcohol as the starting material and sodium periodate as oxidant:
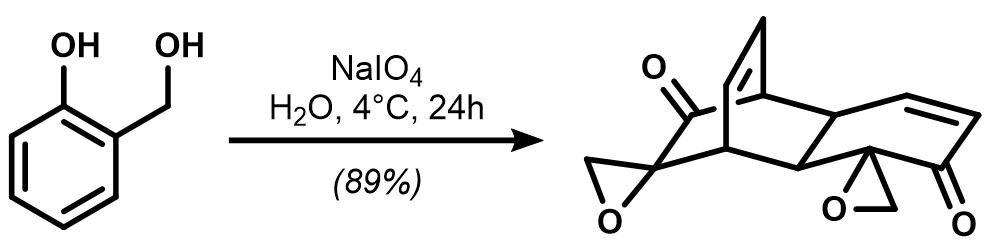
Propose a mechanism for this oxidative dimerization reaction and draw its intermediates. (Hint: the oxidation step takes place first.)
For reference:
Bergner, M., Duquette, D. C., Chio, L., & Stoltz, B. M. (2015).
Exceedingly Efficient Synthesis of (±)-Grandifloracin and Acylated Analogues.
Organic Letters, 17(12), 3008–3010.
doi: 10.1021/acs.orglett.5b01292